The Mississippi center for Advanced Medicine is recruiting Hemophilia A males to understand a new factor VIII gene transfer method.
Patients Must Be:
- Male 18 years or older with Hemophilia A
- Factor VIII deficiency (≤ 2%)
- On-demand therapy or prophylaxis with history of bleeding
- Available for study-related visits for about 12 months
ClinicalTrials.gov Identifier: NCT03003533
Description:
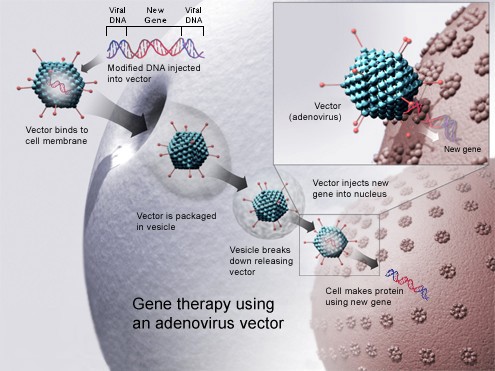
Since the factor VIII gene was discovered, scientists have been working on a way to transfer the factor VIII gene into hemophilia patients’ cells to produce factor VIII protein. In fact, the Sponsor of this study received “breakthrough therapy designation” of their Hemophilia B gene transfer investigational study product from the regulator. In this research study, SPK-8011 (the study product) uses a modified recombinant AAV "vector" (adeno-associated virus) to transfer factor VIII gene into males with Hemophilia A. The study product is injected into the blood-stream by an intravenous (IV) infusion, then travels to the liver where factor VIII protein is made. The factor VIII protein is then released into the bloodstream. If the production of factor VIII by the liver results in high enough levels in the bloodstream, the number of bleeding events may be reduced; however, there are also some risks associated with the study. This research study is to determine if factor VIII gene transfer is safe for the treatment of Hemophilia A.
Can I participate?
Yes, you may be elgible if you meet the following criteria:
- Adult male 18 years or older with hemophilia A (FIX level is 2% or less)
- Have a history of bleeding that requires prophylaxis or on demand therapy
- No history of inhibitor to factor VIII or allergic reaction to factor VIII products
- Don’t have active hepatitis B or C (eligible if treated and clear for at least 6 months)
- Not on antiviral therapy for Hepatitis B or C
- HIV positive is allowed if on effective treatment
- Don’t have severe liver disease
- Available for study-related visits for about 12 months
What is involved?
- A screening phase that may last up to several weeks
- The infusion day may last up to 6 hours. On the infusion day, the study volunteer will have an intravenous (IV) infusion of his regular factor VIII product for 10 minutes then the infusion of the gene transfer study drug for about an hour.
- After the infusion, he will have follow-up visits* up to 52 weeks.
- Most visits will include physical exams, questionnaire, blood draws, and other tests (ranging from 15 minutes to 60 minutes per visit).
* There is flexibility regarding the location of follow-up visits after the study drug infusion and some visits can be arranged by mobile phlebotomists for your convenience (please inquire for more details.)
How will this study be different than previous hemophilia studies?
- AAV vector used in this study was modified to have a higher attraction to the liver, and to minimize immune response and AAV inhibitor formation.
- This is the first AAV-hemophilia A gene transfer study done in the United States.
For inquiry about this study, please contact: Spencer Sullivan, MD at [email protected] or (601) 499-0935.